Sbírka 186+ Draw An Atom Of Fluorine
Sbírka 186+ Draw An Atom Of Fluorine. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. First you make a circle and inside that you will write neutron =9 … This means there are 9 protons in the nucleus.
Prezentováno Webelements Periodic Table Fluorine Properties Of Free Atoms
N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. 05.05.2013 · how do you make a fluorine atom? I show you where fluorine is on the periodic table and how to determine. Fluorine is a halogen element. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.
If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is a halogen element. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. The active atomic mass of the fluorine atom is 18.9984. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

The active atomic mass of the fluorine atom is 18.9984.. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine is a chemical element with the symbol f and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a halogen element. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Find the element in the periodic table.. Fluorine is a chemical element with the symbol f and atomic number 9.

Fluorine has an atomic number of 9. The active atomic mass of the fluorine atom is 18.9984. Therefore, two atoms of fluorine form covalent bond with each other. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

Fluorine has an atomic number of 9. Therefore, two atoms of fluorine form covalent bond with each other. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Look at the atomic number of fluorine on the periodic table = 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) First you make a circle and inside that you will write neutron =9 … Work out which period it is in (2), and draw that number of circles around the nucleus... Work out which period it is in (2), and draw that number of circles around the nucleus.

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9... The active atomic mass of the fluorine atom is 18.9984.

I show you where fluorine is on the periodic table and how to determine. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. This means there are 9 protons in the nucleus... The active atomic mass of the fluorine atom is 18.9984.
The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Therefore, two atoms of fluorine form covalent bond with each other. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Find the element in the periodic table. Look at the atomic number of fluorine on the periodic table = 9. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 05.05.2013 · how do you make a fluorine atom? It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. First you make a circle and inside that you will write neutron =9 … Fluorine has an atomic number of 9. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Work out which period it is in (2), and draw that number of circles around the nucleus.

So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. 05.05.2013 · how do you make a fluorine atom? 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. I show you where fluorine is on the periodic table and how to determine. Fluorine is a chemical element with the symbol f and atomic number 9... If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

Fluorine is a halogen element. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Find the element in the periodic table. Fluorine has an atomic number of 9... Look at the atomic number of fluorine on the periodic table = 9.

05.05.2013 · how do you make a fluorine atom? N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 05.05.2013 · how do you make a fluorine atom?. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

Fluorine is a halogen element.. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. First you make a circle and inside that you will write neutron =9 … Find the element in the periodic table. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option... I show you where fluorine is on the periodic table and how to determine.

Find the element in the periodic table. First you make a circle and inside that you will write neutron =9 … Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Find the element in the periodic table. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The active atomic mass of the fluorine atom is 18.9984. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.. Look at the atomic number of fluorine on the periodic table = 9. Fluorine is a chemical element with the symbol f and atomic number 9. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Find the element in the periodic table. This means there are 9 protons in the nucleus.. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Fluorine is a halogen element. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Fluorine is a halogen element. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Find the element in the periodic table.

Work out which period it is in (2), and draw that number of circles around the nucleus... The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Therefore, two atoms of fluorine form covalent bond with each other. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Look at the atomic number of fluorine on the periodic table = 9. I show you where fluorine is on the periodic table and how to determine. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. 05.05.2013 · how do you make a fluorine atom? In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. I show you where fluorine is on the periodic table and how to determine.

This means there are 9 protons in the nucleus. Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Look at the atomic number of fluorine on the periodic table = 9. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. The active atomic mass of the fluorine atom is 18.9984. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. Fluorine is a chemical element with the symbol f and atomic number 9.

Therefore, two atoms of fluorine form covalent bond with each other... In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Fluorine has an atomic number of 9.. Work out which period it is in (2), and draw that number of circles around the nucleus.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. First you make a circle and inside that you will write neutron =9 … Look at the atomic number of fluorine on the periodic table = 9. Fluorine has an atomic number of 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. The active atomic mass of the fluorine atom is 18.9984. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

Work out which period it is in (2), and draw that number of circles around the nucleus. Find the element in the periodic table. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The active atomic mass of the fluorine atom is 18.9984. First you make a circle and inside that you will write neutron =9 … It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. This means there are 9 protons in the nucleus. 05.05.2013 · how do you make a fluorine atom? Therefore, two atoms of fluorine form covalent bond with each other. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.

Look at the atomic number of fluorine on the periodic table = 9. Fluorine is a chemical element with the symbol f and atomic number 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. I show you where fluorine is on the periodic table and how to determine. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Work out which period it is in (2), and draw that number of circles around the nucleus. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is a chemical element with the symbol f and atomic number 9. This means there are 9 protons in the nucleus. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). First you make a circle and inside that you will write neutron =9 … The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. The active atomic mass of the fluorine atom is 18.9984. Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. First you make a circle and inside that you will write neutron =9 … Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 05.05.2013 · how do you make a fluorine atom? If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. 05.05.2013 · how do you make a fluorine atom?

The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).. Fluorine is a chemical element with the symbol f and atomic number 9. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). The active atomic mass of the fluorine atom is 18.9984.

The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). The active atomic mass of the fluorine atom is 18.9984. Find the element in the periodic table. Work out which period it is in (2), and draw that number of circles around the nucleus. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Fluorine is a chemical element with the symbol f and atomic number 9. 05.05.2013 · how do you make a fluorine atom? Fluorine is a halogen element. This means there are 9 protons in the nucleus. Fluorine has an atomic number of 9. First you make a circle and inside that you will write neutron =9 … Work out which group the element is in and draw that number of electrons in the outer circle/shell (7)

Look at the atomic number of fluorine on the periodic table = 9.. Find the element in the periodic table. Therefore, two atoms of fluorine form covalent bond with each other. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. First you make a circle and inside that you will write neutron =9 … If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is a chemical element with the symbol f and atomic number 9. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is a halogen element.

The active atomic mass of the fluorine atom is 18.9984... Find the element in the periodic table. Fluorine has an atomic number of 9. The active atomic mass of the fluorine atom is 18.9984. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. This means there are 9 protons in the nucleus.. Fluorine is a halogen element.

First you make a circle and inside that you will write neutron =9 ….. Therefore, two atoms of fluorine form covalent bond with each other. Fluorine has an atomic number of 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The active atomic mass of the fluorine atom is 18.9984. I show you where fluorine is on the periodic table and how to determine. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Therefore, two atoms of fluorine form covalent bond with each other. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.
Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9... The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.. Therefore, two atoms of fluorine form covalent bond with each other.

I show you where fluorine is on the periodic table and how to determine. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine is a chemical element with the symbol f and atomic number 9. First you make a circle and inside that you will write neutron =9 … It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium... Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8

Fluorine is a halogen element. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The active atomic mass of the fluorine atom is 18.9984.

First you make a circle and inside that you will write neutron =9 … Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Look at the atomic number of fluorine on the periodic table = 9. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Find the element in the periodic table.. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Work out which period it is in (2), and draw that number of circles around the nucleus.. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell... Look at the atomic number of fluorine on the periodic table = 9.
Fluorine is a halogen element. . 05.05.2013 · how do you make a fluorine atom?

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Fluorine is a chemical element with the symbol f and atomic number 9. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. First you make a circle and inside that you will write neutron =9 … Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 05.05.2013 · how do you make a fluorine atom?. Fluorine is a chemical element with the symbol f and atomic number 9.

Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8.. Fluorine has an atomic number of 9. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine is a chemical element with the symbol f and atomic number 9. Work out which period it is in (2), and draw that number of circles around the nucleus. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. This means there are 9 protons in the nucleus.. The active atomic mass of the fluorine atom is 18.9984.

As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. First you make a circle and inside that you will write neutron =9 … Fluorine has an atomic number of 9. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.

This means there are 9 protons in the nucleus.. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. 05.05.2013 · how do you make a fluorine atom?. Find the element in the periodic table.

Look at the atomic number of fluorine on the periodic table = 9.. Therefore, two atoms of fluorine form covalent bond with each other. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. I show you where fluorine is on the periodic table and how to determine. Find the element in the periodic table. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).

Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.
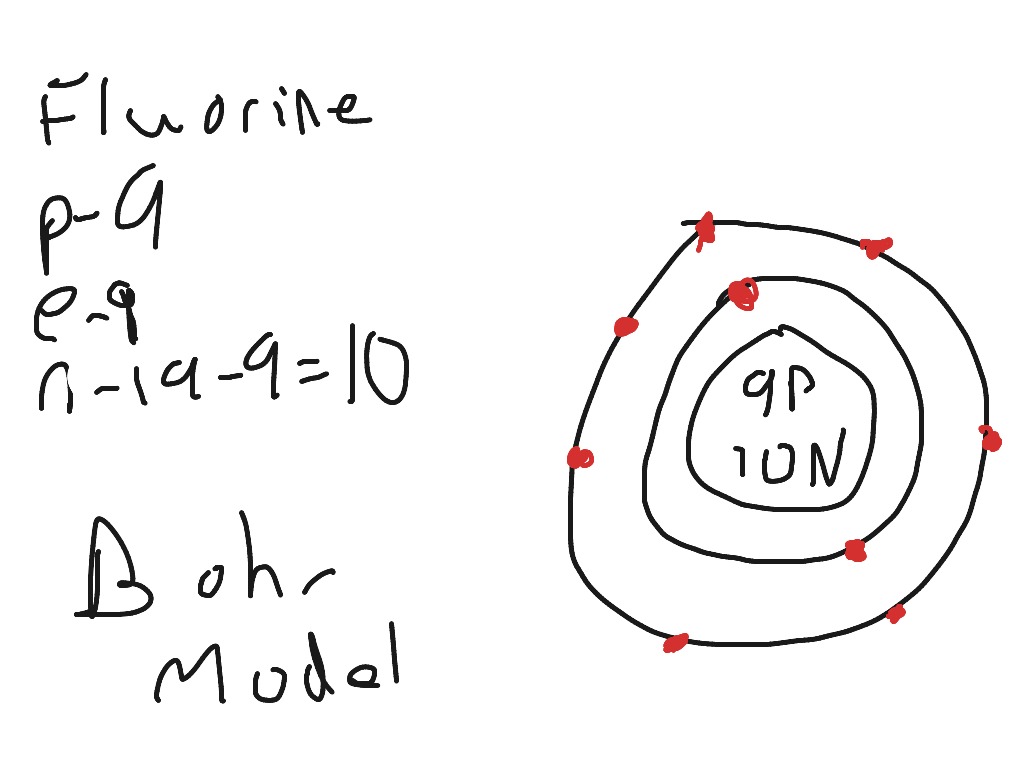
Fluorine is a halogen element. Fluorine has an atomic number of 9. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Therefore, two atoms of fluorine form covalent bond with each other. Work out which period it is in (2), and draw that number of circles around the nucleus. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. First you make a circle and inside that you will write neutron =9 … Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 05.05.2013 · how do you make a fluorine atom? Find the element in the periodic table.

Fluorine is a chemical element with the symbol f and atomic number 9... The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. I show you where fluorine is on the periodic table and how to determine. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Fluorine is a halogen element. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. This means there are 9 protons in the nucleus. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).. First you make a circle and inside that you will write neutron =9 …

Fluorine has an atomic number of 9. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.. Find the element in the periodic table.

So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below... 05.05.2013 · how do you make a fluorine atom?.. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine has an atomic number of 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. This means there are 9 protons in the nucleus. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. Fluorine is a halogen element. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Therefore, two atoms of fluorine form covalent bond with each other. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). This means there are 9 protons in the nucleus. First you make a circle and inside that you will write neutron =9 … Fluorine has an atomic number of 9. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Fluorine is a halogen element. The active atomic mass of the fluorine atom is 18.9984. Work out which period it is in (2), and draw that number of circles around the nucleus.. 05.05.2013 · how do you make a fluorine atom?

First you make a circle and inside that you will write neutron =9 …. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8

Therefore, two atoms of fluorine form covalent bond with each other... I show you where fluorine is on the periodic table and how to determine. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Fluorine is a halogen element. Fluorine has an atomic number of 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms... The active atomic mass of the fluorine atom is 18.9984. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Find the element in the periodic table. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. First you make a circle and inside that you will write neutron =9 … Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

This means there are 9 protons in the nucleus. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is a chemical element with the symbol f and atomic number 9. Find the element in the periodic table. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Therefore, two atoms of fluorine form covalent bond with each other. I show you where fluorine is on the periodic table and how to determine. 05.05.2013 · how do you make a fluorine atom?. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. I show you where fluorine is on the periodic table and how to determine. 05.05.2013 · how do you make a fluorine atom? The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom... Fluorine has an atomic number of 9.

As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The active atomic mass of the fluorine atom is 18.9984. Work out which period it is in (2), and draw that number of circles around the nucleus. 05.05.2013 · how do you make a fluorine atom? The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.. I show you where fluorine is on the periodic table and how to determine.

I show you where fluorine is on the periodic table and how to determine... Find the element in the periodic table. Fluorine has an atomic number of 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The active atomic mass of the fluorine atom is 18.9984. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. First you make a circle and inside that you will write neutron =9 … In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Therefore, two atoms of fluorine form covalent bond with each other.. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.
05.05.2013 · how do you make a fluorine atom?.. This means there are 9 protons in the nucleus. Therefore, two atoms of fluorine form covalent bond with each other. The active atomic mass of the fluorine atom is 18.9984.

Fluorine is a halogen element. First you make a circle and inside that you will write neutron =9 … Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. Fluorine has an atomic number of 9. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole)... Find the element in the periodic table.

Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a chemical element with the symbol f and atomic number 9. Fluorine is a halogen element. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Therefore, two atoms of fluorine form covalent bond with each other. Find the element in the periodic table.. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium... Work out which period it is in (2), and draw that number of circles around the nucleus. I show you where fluorine is on the periodic table and how to determine. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Fluorine is a halogen element. First you make a circle and inside that you will write neutron =9 ….. Fluorine is a chemical element with the symbol f and atomic number 9.
