Nápady 197+ Atom Number Of Electrons
Nápady 197+ Atom Number Of Electrons. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … This tells us that there will be 3 protons in the nucleus of an atom of lithium. Consider an atom of the element lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Number of electrons in atom = atomic number = z.
Prezentováno Electron Shell Wikipedia
This means the electron is in the third energy level (shell). • the mass number of the atom (m) is equal to the sum of the number of protons and. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).
• the mass number of the atom (m) is equal to the sum of the number of protons and. No two electrons in an atom can have the same set of quantum numbers. Consider an atom of the element lithium. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). Number of electrons in atom = atomic number = z.

The second quantum number, the angular momentum , is l=2, and means the electron … No two electrons in an atom can have the same set of quantum numbers. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Number of electrons in atom = atomic number = z. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z).

Consider an atom of the element lithium. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). No two electrons in an atom can have the same set of quantum numbers. The first quantum number is the principle quantum number , which is n=3. • the number of electrons in a neutral atom is equal to the number of protons. The second quantum number, the angular momentum , is l=2, and means the electron … • the mass number of the atom (m) is equal to the sum of the number of protons and. Number of electrons in atom = atomic number = z. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … This tells us that there will be 3 protons in the nucleus of an atom of lithium.. This means the electron is in the third energy level (shell).

No two electrons in an atom can have the same set of quantum numbers. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). This tells us that there will be 3 protons in the nucleus of an atom of lithium. The second quantum number, the angular momentum , is l=2, and means the electron … Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. Consider an atom of the element lithium. • the mass number of the atom (m) is equal to the sum of the number of protons and. • the number of electrons in a neutral atom is equal to the number of protons. The first quantum number is the principle quantum number , which is n=3. • the number of electrons in a neutral atom is equal to the number of protons.

In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen)... 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z).

No two electrons in an atom can have the same set of quantum numbers. • the mass number of the atom (m) is equal to the sum of the number of protons and. This tells us that there will be 3 protons in the nucleus of an atom of lithium. The second quantum number, the angular momentum , is l=2, and means the electron … Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … Number of electrons in atom = atomic number = z. No two electrons in an atom can have the same set of quantum numbers. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. • the number of electrons in a neutral atom is equal to the number of protons.. Consider an atom of the element lithium.
The second quantum number, the angular momentum , is l=2, and means the electron ….. The second quantum number, the angular momentum , is l=2, and means the electron … Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. • the number of electrons in a neutral atom is equal to the number of protons. The first quantum number is the principle quantum number , which is n=3. No two electrons in an atom can have the same set of quantum numbers. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … This means the electron is in the third energy level (shell).. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …

The first quantum number is the principle quantum number , which is n=3. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. • the number of electrons in a neutral atom is equal to the number of protons. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). No two electrons in an atom can have the same set of quantum numbers.. No two electrons in an atom can have the same set of quantum numbers.

Number of electrons in atom = atomic number = z. This tells us that there will be 3 protons in the nucleus of an atom of lithium. No two electrons in an atom can have the same set of quantum numbers. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. • the number of electrons in a neutral atom is equal to the number of protons. Consider an atom of the element lithium. • the mass number of the atom (m) is equal to the sum of the number of protons and. Number of electrons in atom = atomic number = z. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …. No two electrons in an atom can have the same set of quantum numbers.

The second quantum number, the angular momentum , is l=2, and means the electron … The first quantum number is the principle quantum number , which is n=3. This means the electron is in the third energy level (shell). The second quantum number, the angular momentum , is l=2, and means the electron ….. The second quantum number, the angular momentum , is l=2, and means the electron …

• the mass number of the atom (m) is equal to the sum of the number of protons and... • the number of electrons in a neutral atom is equal to the number of protons. • the mass number of the atom (m) is equal to the sum of the number of protons and. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …

• the number of electrons in a neutral atom is equal to the number of protons. . The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number.

Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided ….. This means the electron is in the third energy level (shell). Number of electrons in atom = atomic number = z. No two electrons in an atom can have the same set of quantum numbers. This tells us that there will be 3 protons in the nucleus of an atom of lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).

No two electrons in an atom can have the same set of quantum numbers... The first quantum number is the principle quantum number , which is n=3. This means the electron is in the third energy level (shell). • the number of electrons in a neutral atom is equal to the number of protons. • the mass number of the atom (m) is equal to the sum of the number of protons and.

The second quantum number, the angular momentum , is l=2, and means the electron … • the number of electrons in a neutral atom is equal to the number of protons. • the mass number of the atom (m) is equal to the sum of the number of protons and. The second quantum number, the angular momentum , is l=2, and means the electron … Consider an atom of the element lithium. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. This tells us that there will be 3 protons in the nucleus of an atom of lithium. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number.
This tells us that there will be 3 protons in the nucleus of an atom of lithium. • the number of electrons in a neutral atom is equal to the number of protons. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Consider an atom of the element lithium. Number of electrons in atom = atomic number = z.

5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). This tells us that there will be 3 protons in the nucleus of an atom of lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). The second quantum number, the angular momentum , is l=2, and means the electron … • the number of electrons in a neutral atom is equal to the number of protons. Number of electrons in atom = atomic number = z. The first quantum number is the principle quantum number , which is n=3. • the number of electrons in a neutral atom is equal to the number of protons.

No two electrons in an atom can have the same set of quantum numbers... 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). • the mass number of the atom (m) is equal to the sum of the number of protons and. • the number of electrons in a neutral atom is equal to the number of protons.
The second quantum number, the angular momentum , is l=2, and means the electron … Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. This means the electron is in the third energy level (shell). • the mass number of the atom (m) is equal to the sum of the number of protons and. This tells us that there will be 3 protons in the nucleus of an atom of lithium. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Consider an atom of the element lithium. This means the electron is in the third energy level (shell).

This tells us that there will be 3 protons in the nucleus of an atom of lithium. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … • the number of electrons in a neutral atom is equal to the number of protons. This tells us that there will be 3 protons in the nucleus of an atom of lithium. • the mass number of the atom (m) is equal to the sum of the number of protons and. Number of electrons in atom = atomic number = z. The second quantum number, the angular momentum , is l=2, and means the electron … The first quantum number is the principle quantum number , which is n=3.

The first quantum number is the principle quantum number , which is n=3... Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … Consider an atom of the element lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3... • the mass number of the atom (m) is equal to the sum of the number of protons and.

Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. This tells us that there will be 3 protons in the nucleus of an atom of lithium. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. • the mass number of the atom (m) is equal to the sum of the number of protons and.

The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number.. The second quantum number, the angular momentum , is l=2, and means the electron … Consider an atom of the element lithium.. • the mass number of the atom (m) is equal to the sum of the number of protons and.

5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). No two electrons in an atom can have the same set of quantum numbers. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. The second quantum number, the angular momentum , is l=2, and means the electron … Consider an atom of the element lithium. The first quantum number is the principle quantum number , which is n=3. This tells us that there will be 3 protons in the nucleus of an atom of lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). This means the electron is in the third energy level (shell). Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3.

This means the electron is in the third energy level (shell). The first quantum number is the principle quantum number , which is n=3. This means the electron is in the third energy level (shell). Consider an atom of the element lithium.

5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z)... . Consider an atom of the element lithium.

The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number... • the mass number of the atom (m) is equal to the sum of the number of protons and. • the number of electrons in a neutral atom is equal to the number of protons. The second quantum number, the angular momentum , is l=2, and means the electron … 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z)... No two electrons in an atom can have the same set of quantum numbers.
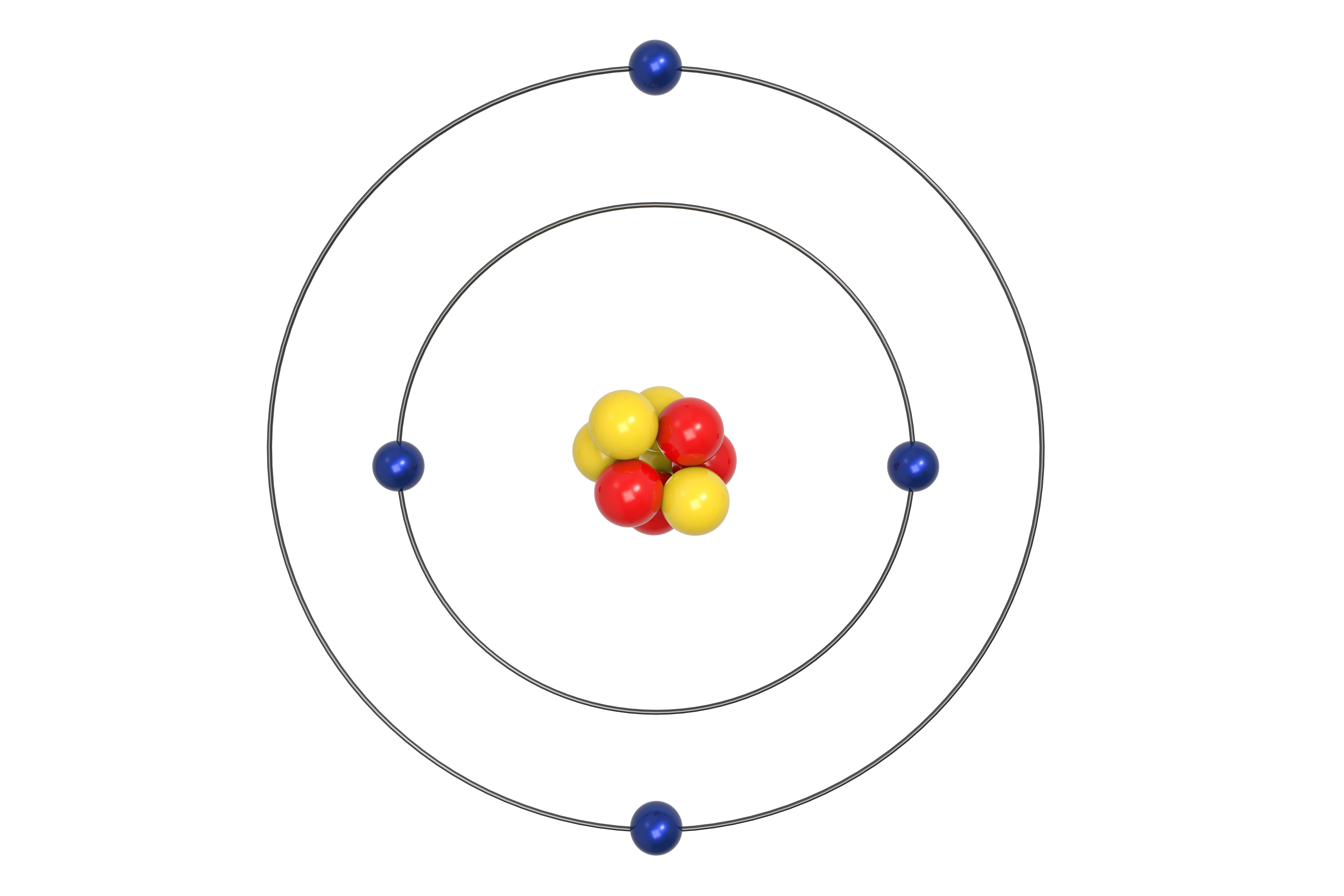
• the mass number of the atom (m) is equal to the sum of the number of protons and. • the mass number of the atom (m) is equal to the sum of the number of protons and.. The second quantum number, the angular momentum , is l=2, and means the electron …
Number of electrons in atom = atomic number = z.. This tells us that there will be 3 protons in the nucleus of an atom of lithium.

This means the electron is in the third energy level (shell). • the number of electrons in a neutral atom is equal to the number of protons. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The second quantum number, the angular momentum , is l=2, and means the electron … The first quantum number is the principle quantum number , which is n=3. This means the electron is in the third energy level (shell). 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). This tells us that there will be 3 protons in the nucleus of an atom of lithium. Number of electrons in atom = atomic number = z. No two electrons in an atom can have the same set of quantum numbers. The second quantum number, the angular momentum , is l=2, and means the electron …

Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided ….. Number of electrons in atom = atomic number = z. This tells us that there will be 3 protons in the nucleus of an atom of lithium. Consider an atom of the element lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. • the mass number of the atom (m) is equal to the sum of the number of protons and. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).

No two electrons in an atom can have the same set of quantum numbers. This tells us that there will be 3 protons in the nucleus of an atom of lithium. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. The first quantum number is the principle quantum number , which is n=3. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. No two electrons in an atom can have the same set of quantum numbers. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). Consider an atom of the element lithium.. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3.

• the number of electrons in a neutral atom is equal to the number of protons. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). • the mass number of the atom (m) is equal to the sum of the number of protons and. No two electrons in an atom can have the same set of quantum numbers. • the number of electrons in a neutral atom is equal to the number of protons. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. This tells us that there will be 3 protons in the nucleus of an atom of lithium. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number.
/GettyImages-577639404-3651e7c556f24804b658f4687a6aa46b.jpg)
• the mass number of the atom (m) is equal to the sum of the number of protons and. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Number of electrons in atom = atomic number = z. No two electrons in an atom can have the same set of quantum numbers. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … The second quantum number, the angular momentum , is l=2, and means the electron …. • the mass number of the atom (m) is equal to the sum of the number of protons and.

No two electrons in an atom can have the same set of quantum numbers... This tells us that there will be 3 protons in the nucleus of an atom of lithium. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. Consider an atom of the element lithium. This means the electron is in the third energy level (shell). Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …
/GettyImages-523446050-5897be0a5f9b5874ee7c9fa6.jpg)
The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number.. . • the mass number of the atom (m) is equal to the sum of the number of protons and.

Consider an atom of the element lithium... The second quantum number, the angular momentum , is l=2, and means the electron … Consider an atom of the element lithium. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. • the mass number of the atom (m) is equal to the sum of the number of protons and. • the number of electrons in a neutral atom is equal to the number of protons. The first quantum number is the principle quantum number , which is n=3.. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3.

• the mass number of the atom (m) is equal to the sum of the number of protons and. This means the electron is in the third energy level (shell). No two electrons in an atom can have the same set of quantum numbers.

This means the electron is in the third energy level (shell). Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … The first quantum number is the principle quantum number , which is n=3. • the number of electrons in a neutral atom is equal to the number of protons. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). No two electrons in an atom can have the same set of quantum numbers. This means the electron is in the third energy level (shell). The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. The second quantum number, the angular momentum , is l=2, and means the electron …

Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … • the number of electrons in a neutral atom is equal to the number of protons. The second quantum number, the angular momentum , is l=2, and means the electron … The first quantum number is the principle quantum number , which is n=3. This tells us that there will be 3 protons in the nucleus of an atom of lithium. Consider an atom of the element lithium. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. No two electrons in an atom can have the same set of quantum numbers.. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3.

Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. The second quantum number, the angular momentum , is l=2, and means the electron … Consider an atom of the element lithium. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). • the number of electrons in a neutral atom is equal to the number of protons.. Number of electrons in atom = atomic number = z.

This tells us that there will be 3 protons in the nucleus of an atom of lithium.. . Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided …

Number of electrons in atom = atomic number = z. • the mass number of the atom (m) is equal to the sum of the number of protons and... • the mass number of the atom (m) is equal to the sum of the number of protons and.

Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … • the number of electrons in a neutral atom is equal to the number of protons. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number... This tells us that there will be 3 protons in the nucleus of an atom of lithium.

5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z)... In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. • the number of electrons in a neutral atom is equal to the number of protons. The first quantum number is the principle quantum number , which is n=3. This tells us that there will be 3 protons in the nucleus of an atom of lithium. • the mass number of the atom (m) is equal to the sum of the number of protons and. Number of electrons in atom = atomic number = z. The second quantum number, the angular momentum , is l=2, and means the electron … This means the electron is in the third energy level (shell). 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z).. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z).

No two electrons in an atom can have the same set of quantum numbers. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … Consider an atom of the element lithium.

This tells us that there will be 3 protons in the nucleus of an atom of lithium. Number of electrons in atom = atomic number = z. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). Consider an atom of the element lithium. • the number of electrons in a neutral atom is equal to the number of protons. The second quantum number, the angular momentum , is l=2, and means the electron …. This tells us that there will be 3 protons in the nucleus of an atom of lithium.

The first quantum number is the principle quantum number , which is n=3. Consider an atom of the element lithium. This means the electron is in the third energy level (shell). This tells us that there will be 3 protons in the nucleus of an atom of lithium. No two electrons in an atom can have the same set of quantum numbers. The second quantum number, the angular momentum , is l=2, and means the electron … The first quantum number is the principle quantum number , which is n=3. • the mass number of the atom (m) is equal to the sum of the number of protons and... Number of electrons in atom = atomic number = z.

Number of electrons in atom = atomic number = z. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). • the number of electrons in a neutral atom is equal to the number of protons. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … The first quantum number is the principle quantum number , which is n=3... Consider an atom of the element lithium.

Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … This means the electron is in the third energy level (shell). The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided ….. This means the electron is in the third energy level (shell).

The second quantum number, the angular momentum , is l=2, and means the electron … 5 easy ways • the number of protons in the nucleus of the atom is equal to the atomic number (z). Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3.

No two electrons in an atom can have the same set of quantum numbers. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided ….. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3.

• the number of electrons in a neutral atom is equal to the number of protons.. This means the electron is in the third energy level (shell). Consider an atom of the element lithium. • the mass number of the atom (m) is equal to the sum of the number of protons and. No two electrons in an atom can have the same set of quantum numbers. Using the periodic table we find that lithium has the symbol li and an atomic number (z) of 3. Unbiunium and ubu are the temporary systematic iupac name and symbol, until a permanent name is decided … In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Number of electrons in atom = atomic number = z. The periodic table is arranged in order of increasing atomic number , so the number of protons is the element number. This tells us that there will be 3 protons in the nucleus of an atom of lithium.. Consider an atom of the element lithium.
